Research Area: Pediatric hematology, oncology and hematopoietic cell&gene therapy
Group Leader
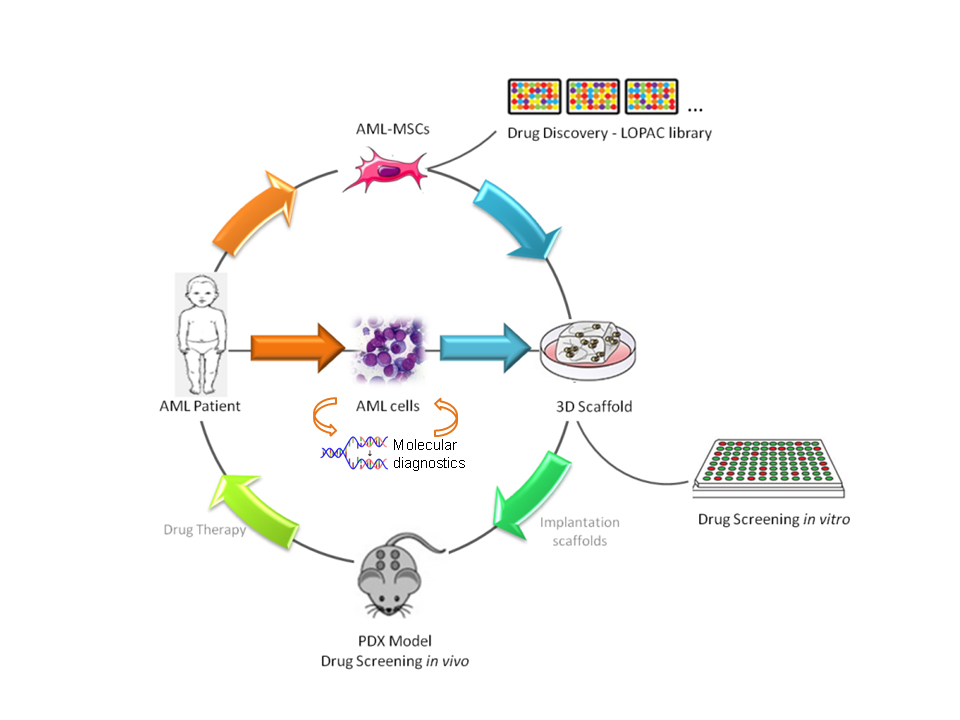
Leukemias account for approximately one third of all pediatric malignancies and remain a leading cause of morbidity and mortality in children and adolescents. Acute myeloid leukemia (AML) represents around 20% of cases of acute leukemia in childhood. Even if the outcome of the affected children has significantly improved over the past 30 years, reaching up to 60% of survival at 8 years in most of recently concluded clinical trials, no new or specific drugs have been recently introduced. Notwithstanding complete remission rates of 85–90%, disease recurrence still represents the main cause of treatment failure. Our research aims at the identification of new genetic aberrations and mutations with prognostic value to be used as biomarkers to improve diagnosis and risk stratification of patients and their treatment response by employing tailored adapted therapies. To this goal, thanks to large retrospective studies, we characterized a large number of new AML mutations at diagnosis with relevant prognostic role and established new quantitative molecular assays for monitoring minimal molecular disease, increasing the ability to look after patients during their follow up. This has relevant implications in disease management, specifically before hematopoietic cell transplantation. For most of the identified new mutations we also set up functional studies deepening into the mechanism of leukemogenesis. The identification of structural alterations, such as inter- and intra-chromosomal rearrangements, remains the most frequent mechanism of mutagenesis occurring in pediatric AML, but for a large proportion of patients (up to 50%) a genetic biomarker is not present at diagnosis. Thus, we interpreted the intimate connection between the dysregulation of gene expression and malignant transformation, and highlighted the importance of investigating key players in the regulation of gene expression to shed light on leukemogenesis and to define new therapeutic approaches for AML. Our work is based on coding and non-coding genes, such as lncRNAs and microRNAs, to dissect the key pathogenic mechanisms of disease onset and recurrence, looking for new targets. We employed integrated high-throughput approaches looking at the DNA sequencing and methylation, transcriptome of coding and non-coding genes, and proteome status of patients to be then recapitulated in several in vitro, 2D and 3D, and in vivo models, i.e. patient-derived xenografts (PDX), to create new treatment opportunities. This translational research approach aims at understanding cancer transformation mechanisms, with particular interest in the identification of driver oncogenes and activated pathways, for possibly developing promising alternatives or complementary therapeutics to current chemotherapy, particularly in children who experience relapse. To this goal, we employ a repositioning strategy of old drugs by using high-throughput drug screening of commercial libraries with FDA-approved compounds, as well as targeted therapy approaches following the main international Consortia of the Leukemia and Lymphoma society for novel drugs included in clinical trials. More recently, we also became interested in cancer cell metabolism and mitochondrial targeting, tin he use of nanoparticles for non-coding RNA delivery, and in tumor specific antigen identification for the development of novel targeted immune-therapy approaches specific for AML. Finally, we recently joined the Children’s Liver Tumor European Research Network – ChiLTERN, a H2020 project aiming at creating innovative in vivo model to identify new anticancer drugs or combinations of drugs for the treatment of patients with high-risk hepatoblastoma facing disease recurrence or chemotherapeutic treatment-failure. This activity would provide proof of concept for patients enrolled in the PHITT trial which is a collaborative study involving major clinical European groups running pediatric liver tumor trials.
Group Members
Claudia Tregnago, Post-Doctoral Fellow
Elena Porcù, Post-Doctoral Fellow
Giulia Borile, Post Doctoral Fellow
Giulia Borella, Post-Doctoral Fellow
Maddalena Benetton, Post Doctoral Fellow
Ambra Da Ros, PhD Student
Anna Marchetti, Graduate fellow
Selected Publications
Tregnago C, Da Ros A, Porcù E, Benetton M, Simonato M, Simula L, Borella G, Polato K, Minuzzo S, Borile G, Cogo P, Campello S, Massi A, Romagnoli R, Buldini B, Locatelli F, Pigazzi M. Thioridazine requires calcium influx to induce MLL-AF6-rearranged AML cell death. Blood Adv. 2020 Sep 22;4(18):4417-4429;
Simula L, Corrado M, Accordi B, Di Rita A, Nazio F, Antonucci Y, Di Daniele, A, Caicci F, Caruana I, Soriano ME, Pigazzi M, Locatelli F, Cecconi F, Campello, S. JNK1 and ERK1/2 modulate lymphocyte homeostasis via BIM and DRP1 upon AICD induction. Cell Death Differ. 2020 Oct;27(10):2749-2767;
Noort S, Zimmermann M, Reinhardt D, Cuccuini W, Pigazzi M, Smith J, Ries RE, Alonzo TA, Hirsch B, Tomizawa D, Locatelli F, Gruber TA, Raimondi S, Sonneveld E, Cheuk DK, Dworzak M, Stary J, Abrahamsson J, Arad-Cohen N, Czogala M, De Moerloose B, Hasle H, Meshinchi S, van den Heuvel-Eibrink M, Zwaan CM. Prognostic impact of t(16;21)(p11;q22) and t(16;21)(q24;q22) in pediatric AML: a retrospective study by the I-BFM Study Group. Blood. 2018:11;132(15):1584-1592;
Zampini M, Tregnago C, Bisio V, Simula L, Borella G, Manara E, Zanon C, Zonta F, Serafin V, Accordi B, Campello S, Buldini B, Pession A, Locatelli F, Basso G, Pigazzi M. Epigenetic heterogeneity affects the risk of relapse in children with t(8;21)RUNX1-RUNX1T1-rearranged AML. Leukemia. 2018 May;32(5):1124-1134;
de Rooij JD, Branstetter C, Ma J, Li Y, Walsh MP, Cheng J, Obulkasim A, Dang J, Easton J, Verboon LJ, Mulder HL, Zimmermann M, Koss C, Gupta P, Edmonson M, Rusch M, Lim JY, Reinhardt K, Pigazzi M, Song G, Yeoh AE, Shih LY, Liang DC, Halene S, Krause DS, Zhang J, Downing JR, Locatelli F, Reinhardt D, van den Heuvel-Eibrink MM, Zwaan CM, Fornerod M, Gruber TA. Pediatric non-Down syndrome acute megakaryoblastic leukemia is characterized by distinct genomic subsets with varying outcomes. Nat Genet. 2017 Mar;49(3):451-456;
Bisio V, Zampini M, Tregnago C, Manara E, Salsi V, Di Meglio A, Masetti R, Togni M, Di Giacomo D, Minuzzo S, Leszl A, Zappavigna V, Rondelli R, Mecucci C, Pession A, Locatelli F, Basso G, Pigazzi M. NUP98-fusion transcripts characterize different biological entities within acute myeloid leukemia: a report from the AIEOP-AML group. Leukemia. 2017 Apr;31(4):974-977;
Manara E, Basso G, Zampini M, Buldini B, Tregnago C, Rondelli R, Masetti R, Bisio V, Frison M, Polato K, Cazzaniga G, Menna G, Fagioli F, Merli P, Biondi A, Pession A, Locatelli F, Pigazzi M. Characterization of children with FLT3-ITD acute myeloid leukemia: a report from the AIEOP AML-2002 study group. Leukemia. 2017 Jan;31(1):18-25;
Tregnago C, Manara E, Zampini M, Bisio V, Borga C, Bresolin S, Aveic S, Germano G, Basso G, Pigazzi M. CREB engages C/EBPδ to initiate leukemogenesis. Leukemia. 2016 Sep;30(9):1887-96;
Palanichamy JK, Tran TM, Howard JM, Contreras JR, Fernando TR, Sterne-Weiler T, Katzman S, Toloue M, Yan W, Basso G, Pigazzi M, Sanford JR, Rao DS. RNA- binding protein IGF2BP3 targeting of oncogenic transcripts promotes hematopoietic progenitor proliferation. J Clin Invest. 2016 Apr 1;126(4):1495-511;
Manara E, Baron E, Tregnago C, Aveic S, Bisio V, Bresolin S, Masetti R, Locatelli F, Basso G, Pigazzi M. MLL-AF6 fusion oncogene sequesters AF6 into the nucleus to trigger RAS activation in myeloid leukemia. Blood. 2014 Jul 10;124(2):263-72.
Contact

Corso Stati Uniti, 4 F
35127 Padova
Phone: +39 049 9640111
Fax: +39 049 9640101
info@irpcds.org
Orario di apertura: lun-ven 8:30 – 17:30