Research Area: Immunology and Neuroimmunology
Group Leader
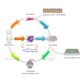
Reactive oxygen species (ROS) are well known to be fundamental for macrophages to kill invasive microorganisms. Moreover, they have an important role in regulating signal transduction pathways, gene expression and differentiation. Besides NADPH oxidase, mitochondria are gaining increasing relevance as a source of ROS in immune cells, although the exact sites of formation are only partially elucidated. Monoamine oxidase (MAO) is a relevant source of hydrogen peroxide in mitochondria, generated by oxidative deamination of biogenic amines. Since this enzyme has been scarcely characterized in phagocytic cells, we aimed at clarifying whether it plays a role in the differentiation and activation of macrophages.
Our findings show that oxidative stress induced by MAO activity plays a crucial role in inflammasome activation in acute and chronic inflammation. Inflammasomes represent protective weapons against pathogens and cellular damage, though their uncontrolled activation drives progression of inflammatory, metabolic, and neurodegenerative disorders. Several signals activate the NLRP3 inflammasome and a few studies reported that mitochondrial reactive oxygen species (ROS) are involved in this process. However, it is still unclear what is the specific role of mitochondrial ROS in NLRP3 triggering and, most importantly, which is their specific source. Mechanistically, MAO-B-dependent ROS formation caused mitochondrial dysfunction and NF-kB induction, resulting in NLRP3 and pro-IL-1B overexpression. Both in vitro and in vivo, MAO-B inhibition by rasagiline prevented IL-1B secretion and MAO-B deficient mice showed impaired response to LPS-mediated endotoxemia. Importantly, in a Duchenne dystrophy model, rasagiline administration reduced inflammasome activation in muscle-infiltrating macrophages, along with muscle performance recovery. Our findings identify MAO-B as a specific producer of mitochondrial ROS fuelling NLRP3 inflammasome, thereby providing the basis for repurposing MAO-B inhibitors to treat inflammasome-mediated pathologies.
Thus, we are currently investigating whether clinical-grade monoamine oxidase inhibitors can be viable candidates in the treatment of autoinflammatory and autoimmune disorders.
Group Members
Ricardo Sanchez-Rodriguez PostDoctoral Researcher
Eugenia Carraro PhD student
Giorgia Contarini Master student
Selected Publications
Sánchez-Rodríguez R, Munari F, Angioni R, Venegas F, Agnellini A, Castro-Gil MP, Castegna A, Luisetto R, Viola A, Canton M (2020) Targeting monoamine oxidase to dampen NLRP3 inflammasome activation in acute and chronic inflammation. Cell Mol Immunol doi: 10.1038/s41423-020-0441-8.
Castegna A, Gissi R, Menga A, Montopoli M, Favia M, Viola A, Canton M (2020) Pharmacological targets of metabolism in disease: opportunities from macrophages Pharmacol. Ther 210:107521. doi: 10.1016/j.pharmthera.2020.107521.
Costiniti V, Spera I, Menabo R, Palmieri EM, Menga A, Scarcia P, Porcelli V, Gissi R, Castegna A, Canton M (2018) Monoamine oxidase-dependent histamine catabolism accounts for post-ischemic cardiac redox imbalance and injury. Biochim Biophys Acta Mol Basis Dis, 1864:3050-9.
Canton M, Menazza S, Sheeran FL, Polverino De Laureto P, Di Lisa F, And Pepe S (2011) Oxidation of myofibrillar proteins in human heart failure. J Am Coll Cardiol, vol. 57 (3); p. 300-309, ISSN: 0735-1097.
Menazza S, Blaauw B, Tiepolo T, Toniolo L, Braghetta P, Spolaore B, Reggiani C, Di Lisa F, Bonaldo P, Canton M (2010) Oxidative stress by monoamine oxidases is causally involved in myofiber damage in muscular dystrophy. Hum Mol Genet, vol. 19; p. 4207-4215.
Contact

Corso Stati Uniti, 4 F
35127 Padova
Phone: +39 049 9640111
Fax: +39 049 9640101
info@irpcds.org
Orario di apertura: lun-ven 8:30 – 17:30