Research Area: Ematologia e oncologia pediatrica - Terapia genica e trapianto di cellule ematopoietiche
Group Leader
Neuroblastoma (NB) is a pediatric tumor originating from the neural crest–derived sympathoadrenal progenitors. It shows wide-ranging clinical and biological heterogeneity which makes this tumor very hard to treat especially in high-risk (HR) group of patients. In fact, long-term survival for HR patients is still below 50% and various metastatic sites are commonly observed in these patients. Dissemination of tumor cells to the bone marrow (BM) is the most frequent event in HR patients with NB and accounts for about 70% of all metastases. Once BM metastasis is detected, disease progresses rapidly due to an intense growth of NB cells, while patients suffer from extensive bone pain. Therefore, an aggressive course of chemotherapy is often required, although its efficiency is low due to the resistance of NB cells to administered therapy. The lack of standardized in vitro and in vivo models of metastatic NB introduces difficulties in the investigation of the molecular background of aggressive phenotypes and in the selection of new therapeutic strategies. In this context, our main research is focused on the adaptation and enhancement of the innovative in vitro engineering platforms and zebrafish in vivo experimental models of NB that will allow us to study biology of cell spreading within complex 3D microenvironment. This approach will allow a more comprehensive assessment of the molecular events that sustain tumor progression and resistance to the currently proposed therapy options, such as autophagy. Currently, we are investigating the extent to which autophagy activation in NB cells allows their protection from toxic drug insults. By combining autophagy modulators and different anti-tumor drugs we investigate the possibility of introducing autophagy inhibitors as an adjuvant therapy for HR patients affected by NB.
The group is currently working on the following research topics:
i) pharmacology: The research activities of Neuroblastoma Laboratory are focused on better understanding of the biology of NB cells with invasive features. We are particularly interested in elucidating the molecular mechanisms that sustain drug resistance in NB leading to tumor cells’ spreading and disease progression. We have recently revealed the activation of cytoprotective autophagy in NB cells treated with receptor tyrosine kinase inhibitors (Aveic et al, 2016, Aveic et al. 2018, Corallo et al, 2020). We are now assessing the potential of autophagy inhibition in reverting aggressive and resistant NB phenotype. Towards this goal, we are employing 2D and 3D in vitro models and in vivo experimental approaches that should help us in decoding novel therapy options for HR patients with NB.
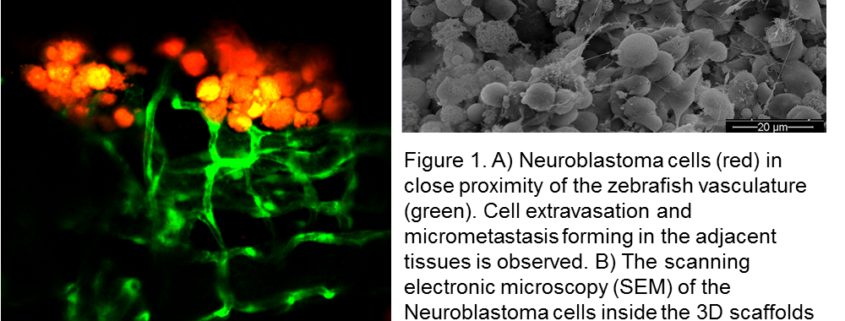
ii) basic research: A further research effort of our group contemplates Lin28B, a gene whose deregulated expression has been correlated with the aggressive NB phenotype. We are currently investigating the role of Lin28B in the neural crest cells migration during early phases of embryonal development of zebrafish larva. In addition, we have recently found that Lin28B overexpressing embryos fail to express detectable levels of markers required for the specification of the sympathoadrenal lineage, suggesting a direct role of Lin28B in the regulation of the peripheral sympathetic nervous system development at the early embryonic stages (Corallo et. al 2020). Moreover, using the zebrafish we have set up the xenotransplantation model of NB cell lines (Aveic et al., 2018) and primary cells in order to obtain PDX models of NB as a potential in vivo platform for pharmacological studies and analysis of cancer cell behavior and extravasation.
iii) innovations: In collaboration with international research group from the RWTH Aachen University Hospital, Aachen, Germany, we are interested in the generation of 3D in vitro models of NB that will allow more extensive studies of biological pathways under complex tumor microenvironment conditions. We are using tumor derived cell lines and ex-vivo primary cells to recapitulate the heterogeneity of NB tumors with an aggressive phenotype.
Group Members
Diana Corallo, Post-doctoral Fellows
Marcella Pantile, Technician
Francesco Minozzi, B.Sc. Master student (in collaboration with the University of Milan)
Selected Publications
Corallo D, Pastorino F, Pantile M, Mariotto E, Caicci F, Viola G, Ponzoni M, Tonini GP, Aveic S. Autophagic flux inhibition enhances cytotoxicity of the receptor tyrosine kinase inhibitor ponatinib. J Exp Clin Cancer Res. 2020; 39(1):195.
Mariotto E, Viola G, Zanon C, Aveic S. A BAG’s life: Every connection matters in cancer. PHARMACOLOGY & THERAPEUTICS, 2020; 209:107498.
Janßen S, Gach S, Kant S, Aveic S, Rütten S, Olschok S, Reisgen U, Fischer H. Enhanced Osteogenic Differentiation of Human Mesenchymal Stromal Cells as Response to Periodical Microstructured Ti6Al4V Surfaces in Journal of Biomedical Materials Research Part B: Applied Biomaterials: 2020, 108(5):2218-2226.
Corallo D, Donadon M, Pantile M, Sidarovich V, Cocchi S, Ori M, De Sarlo M, Candiani S, Frasson C, Distel M, Quattrone A, Zanon C, Basso G, Tonini GP, Aveic S. LIN28B increases neural crest cell migration and leads to transformation of trunk sympathoadrenal precursors. Cell Death Differ. 2020; 27(4):1225-1242.
Aveic S, Davtalab R, Vogt M, Weber W, Buttler-Bücher P, Tonini GP, Fischer H. Calcium phosphate scaffolds with defined interconnecting channel structure provide a mimetic 3D niche for bone marrow metastasized tumor cell growth. Acta Biomater 2019; 88:527-539.
Duarte Campos DF, Bonnin Marquez A, O’Seanain C, Fischer H, Blaeser A, Vogt M, Corallo D, Aveic S. Exploring Cancer Cell Behavior In Vitro in Three-Dimensional Multicellular Bioprintable Collagen-Based Hydrogels. Cancers 2019; 11:180.
Mariotto E, Viola G, Ronca R, Persano L, Aveic S, Bhujwalla ZM, Mori N, Accordi B, Serafin V, López-Cara LC, Bortolozzi R. Choline Kinase Alpha Inhibition by EB-3D Triggers Cellular Senescence, Reduces Tumor Growth and Metastatic Dissemination in Breast Cancer. Cancers (Basel). 2018; 10(10). pii: E391.
Aveic S, Pantile M, Polo P, Sidarovich V, De Mariano M, Quattrone A, Longo L, Tonini GP. Autophagy inhibition improves the cytotoxic effects of receptor tyrosine kinase inhibitors. Cancer Cell Int. 2018; 18:63.
Sidarovich V, De Mariano M, Aveic S, Pancher M, Adami V, Gatto P, Pizzini S, Pasini L, Croce M, Parodi F, Cimmino F, Avitabile M, Emionite L, Cilli M, Ferrini S, Pagano A, Capasso M, Quattrone A, Tonini GP, Longo L. A High-Content Screening of Anticancer Compounds Suggests the Multiple Tyrosine Kinase Inhibitor Ponatinib for Repurposing in Neuroblastoma Therapy. Mol Cancer Ther. 2018; 17(7):1405-1415.
Aveic S, Corallo D, Porcù E, Pantile M, Boso D, Zanon C, Viola G, Sidarovich V, Mariotto E, Quattrone A, Basso G, Tonini GP. TP-0903 inhibits neuroblastoma cell growth and enhances the sensitivity to conventional chemotherapy. Eur J Pharmacol. 2018; 818:435-48.
Contatti

Corso Stati Uniti, 4 F
35127 Padova
Phone: +39 049 9640111
Fax: +39 049 9640101
info@irpcds.org
Orario di apertura: lun-ven 8:30 – 17:30